https://en.sputniknews.africa/20240909/chinas-first-domestically-developed-mpox-vaccine-approved-for-clinical-trials-developers-say-1068212088.html
China's First Domestically Developed Mpox Vaccine Approved for Clinical Trials, Developers Say
China's First Domestically Developed Mpox Vaccine Approved for Clinical Trials, Developers Say
Sputnik Africa
In August, the head of the World Health Organization, Tedros Adhanom Ghebreyesus, declared the outbreak of mpox in southern and Central Africa a public health... 09.09.2024, Sputnik Africa
2024-09-09T17:06+0200
2024-09-09T17:06+0200
2024-09-13T15:02+0200
tedros adhanom ghebreyesus
china
world health organization (who)
mpox (monkeypox)
vaccine
international
disease
healthcare
heath
https://cdn1.img.sputniknews.africa/img/07e7/06/0a/1059834774_0:23:3072:1751_1920x0_80_0_0_0b4ef496022756c8cbbb4eb54aa5d12e.jpg
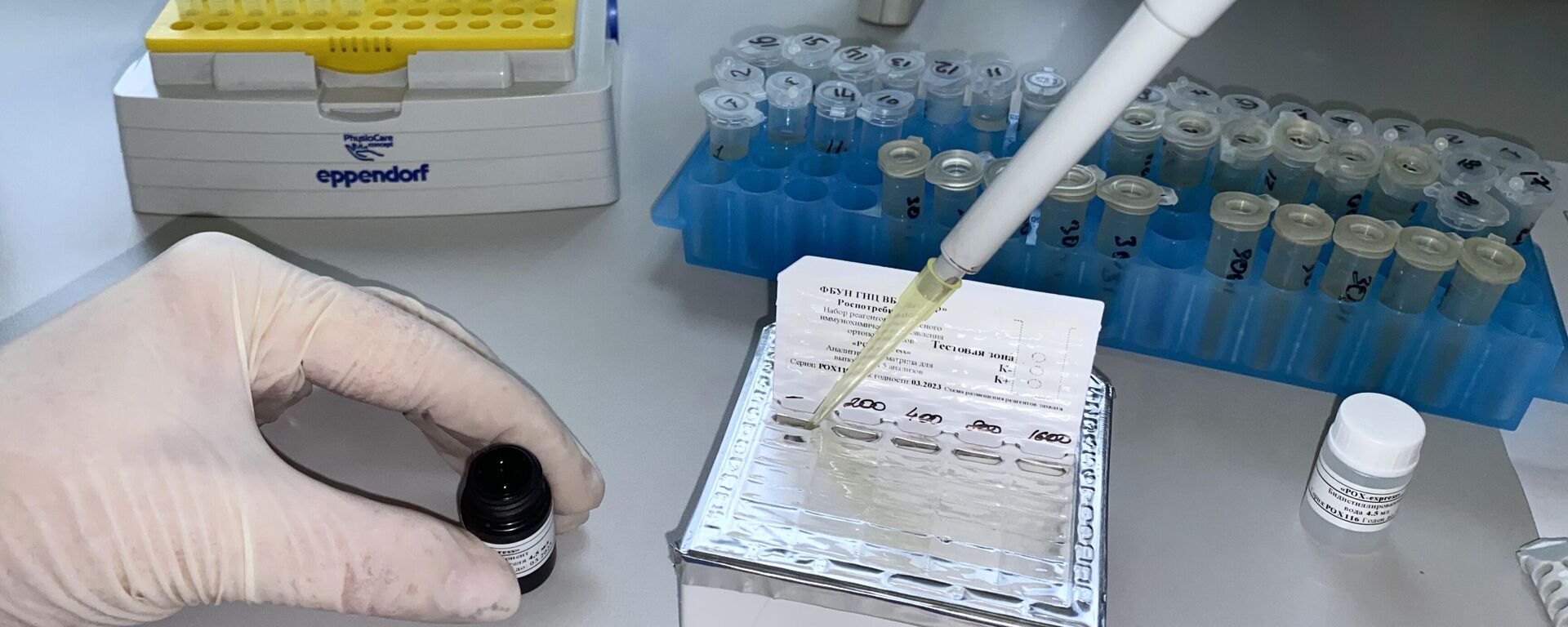
China's first independently developed mpox vaccine has received approval for clinical trials from the country's State Food and Drug Administration on Monday, the Shanghai Institute of Biological Products announced.The live-attenuated MVA mpox vaccine was developed by the Shanghai Institute of Biological Products Co., Ltd., which is run by the China National Pharmaceutical Group Corporation, commonly referred to as Sinopharm.According to a statement published by the company, the vaccine is expected to play an important role in the prevention and control of diseases caused by the mpox virus in China.Mpox, formerly known as monkeypox, is a viral disease that is potentially dangerous for people with weakened immune systems. The infection is accompanied by fever, intoxication, enlargement of the lymph nodes, and subsequent spread of the rash – first in the form of spots that turn into vesicles, after which ulcers form, after which they heal – crusts, and when they fall off – scars. With a mild course, the disease usually disappears on its own and lasts from 14 to 21 days.
https://en.sputniknews.africa/20240905/russias-human-welfare-watchdog-delivers-russian-mpox-tests-batch-to-burundi-1068165826.html
china
Sputnik Africa
feedback@sputniknews.com
+74956456601
MIA „Rossiya Segodnya“
2024
Muhammad Nooh Osman
https://cdn1.img.sputniknews.africa/img/07e7/04/0a/1058467512_0:0:1280:1280_100x100_80_0_0_ec723833bcbfcaed2e21952965ad99e4.jpg
Muhammad Nooh Osman
https://cdn1.img.sputniknews.africa/img/07e7/04/0a/1058467512_0:0:1280:1280_100x100_80_0_0_ec723833bcbfcaed2e21952965ad99e4.jpg
News
en_EN
Sputnik Africa
feedback@sputniknews.com
+74956456601
MIA „Rossiya Segodnya“
Sputnik Africa
feedback@sputniknews.com
+74956456601
MIA „Rossiya Segodnya“
Muhammad Nooh Osman
https://cdn1.img.sputniknews.africa/img/07e7/04/0a/1058467512_0:0:1280:1280_100x100_80_0_0_ec723833bcbfcaed2e21952965ad99e4.jpg
tedros adhanom ghebreyesus, china, world health organization (who), mpox (monkeypox), vaccine, international, disease, healthcare, heath
tedros adhanom ghebreyesus, china, world health organization (who), mpox (monkeypox), vaccine, international, disease, healthcare, heath
China's First Domestically Developed Mpox Vaccine Approved for Clinical Trials, Developers Say
17:06 09.09.2024 (Updated: 15:02 13.09.2024) Muhammad Nooh Osman
Writer/Editor
In August, the head of the World Health Organization, Tedros Adhanom Ghebreyesus, declared the outbreak of mpox in southern and Central Africa a public health emergency of international concern.
China's first independently developed
mpox vaccine has received approval for clinical trials from the country's State Food and Drug Administration on Monday, the Shanghai Institute of Biological Products announced.
The live-attenuated MVA mpox vaccine was developed by the Shanghai Institute of Biological Products Co., Ltd., which is run by the China National Pharmaceutical Group Corporation, commonly referred to as Sinopharm.
According to a statement published by the company, the
vaccine is expected to play an important role in the prevention and control of diseases caused by the mpox virus in China.
Mpox, formerly known as monkeypox, is a viral disease that is potentially dangerous for people with weakened immune systems. The infection is accompanied by fever, intoxication, enlargement of the lymph nodes, and subsequent spread of the rash – first in the form of spots that turn into vesicles, after which ulcers form, after which they heal – crusts, and when they fall off – scars. With a mild course, the disease usually disappears on its own and lasts from 14 to 21 days.